-
Engineering and Architecture
Exams
Colleges
Predictors
Resources
-
Computer Application and IT
Quick Links
Colleges
-
Pharmacy
Colleges
Resources
-
Hospitality and Tourism
Colleges
Resources
Diploma Colleges
-
Competition
Other Exams
Resources
-
School
Exams
Top Schools
Products & Resources
-
Study Abroad
Top Countries
Resources
-
Arts, Commerce & Sciences
Exams
Colleges
Upcoming Events
Resources
-
Management and Business Administration
Colleges & Courses
Predictors
-
Learn
Law Preparation
MBA Preparation
Engineering Preparation
Medical Preparation
-
Online Courses and Certifications
Top Streams
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
Resources
-
Medicine and Allied Sciences
Colleges
Predictors
Resources
-
Law
Resources
Colleges
-
Animation and Design
Exams
Predictors & Articles
Colleges
Resources
-
Media, Mass Communication and Journalism
Colleges
Resources
-
Finance & Accounts
Top Courses & Careers
Colleges
Get Answers to all your Questions


All Questions
An ideal gas undergoes isothermal expansion at constant pressure. During the process :
Option: 1 enthalpy increases but entropy decreases.
Option: 2 enthalpy remains constant but entropy increases.
Option: 3 enthalpy decreases but entropy increases.
Option: 4 Both enthalpy and entropy remain constant.

The major product obtained in the following reaction is :
Option: 1 
Option: 2
Option: 3 
Option: 4 
The major product is represented by the option (D). DIBAL−H reduces esters to aldehydes. It also reduces carboxylic acids to aldehydes.

Crack CUET with india's "Best Teachers"
- HD Video Lectures
- Unlimited Mock Tests
- Faculty Support

The most abundant elements by mass in the body of a healthy human adult are : Oxygen (61.4%); Carbon (22.9%), Hydrogen (10.0%); and Nitrogen (2.6%). The weight (in kg) which a 75 kg person would gain if all atoms are replaced by
atoms is :
Option: 1 7.5
Option: 2 10
Option: 3 15
Option: 4 37.5
Given that
Mass of the person = 75 kg
Mass of 1H1 present in person = 10% of 75 kg = 7.5 kg
Since Mass of 1H2 is double the Mass of 1H1
So, Mass of 1H2 will be in person = 2 X 7.5 kg =15 kg
Thus, increase in weight = 15 - 7.5 = 7.5 kg
Therefore, Option (1) is correct
View Full Answer(1)A plane is inclined at an angle 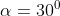 with respect to the horizontal. A particle is projected with a speed
with respect to the horizontal. A particle is projected with a speed 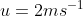 from the base of the plane , making an angle
from the base of the plane , making an angle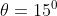 With respect to the plane as shown in the figure . The distance from the base ,at which the particle hits the plane is close to:
With respect to the plane as shown in the figure . The distance from the base ,at which the particle hits the plane is close to: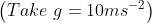

Option: 1 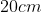
Option: 2 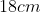
Option: 3 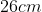
Option: 4 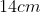
As we know on inclined plane the range is given by
JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE
- #Classification of elements and periodicity in properties
- #Engineering
- #Chemistry
- #Joint Entrance Examination Main
The correct order of the atomic radii of C, Cs, Al and S is :
Option: 1
Option: 2
Option: 3
Option: 4
Periodicity of atomic radius and ionic radius in period -
In a period from left to right the effective nuclear charge increases because the next electron fills in the same shell. So the atomic size decrease.
- wherein
Electronegativity and atomic radius -
The attraction between the outer electrons and the nucleus increases as the atomic radius decreases in a period.
- wherein
Size of atom and ion in a group -
In a group moving from top to the bottom the number of shell increases.So the atomic size increases.
- wherein
As we know that
From Left to right in a period size decreases and when going down the group size increases
Therefore, Option(2) is correct
View Full Answer(1)
- #Classification of elements and periodicity in properties
- #Engineering
- #Chemistry
- #Joint Entrance Examination Main
The IUPAC symbol for the element with atomic number 119 would be:
Option: 1 uue
Option: 2une
Option: 3 unh
Option: 4 uun
Nomenclature of elements with atomic number >100 -
The name is derived directly from the atomic number of the element using the following numerical roots:
0 = nil
1 = un
2 = bi
3 = tri
4 = quad
5 = pent
6 = hex
7 = sept
8 = oct
9 = enn
Eg:
|
Atomic number |
Name |
Symbol |
|
101 |
Mendelevium (Unnilunium) |
Md (Unu) |
|
102 |
Nobelium (Unnilbium) |
No (Unb) |
-
uue
1 1 9
Un Un ennium
Therefore, Option(1) is correct.
View Full Answer(1)NEET 2024 Most scoring concepts
- Just Study 32% of the NEET syllabus and Score up to 100% marks
The dimension of stopping potential in photoelectric effect in units of Planck's constant 'h', speed of light 'c' and Gravitational constant 'G' and ampere A is :
Option: 1
Option: 2
Option: 3
Option: 4
Let
Now,
So,
From here we will get: p-q=1---------(1)
2p+3q+r=2----------(2)
-p-2q-r=-3----------(3)
s=-1-----------(4)
From equation 1, 2,3 and 4 we will get: p=0,q=-1, r=5 and s=-1
So,
The ammonia released on quantitative reaction of 0.6g, urea
with sodium hydroxide
can be neutralised by:
Option: 1 200 ml of 0.2 N HCl
Option: 2200 ml of 0.4 N HCl
Option: 3100 ml of 0.1N HCl
Option: 4100 ml of 0.2N HCl
2 × mole of Urea = mole of ........(1)
mole of = mole of
........(2)
mole of HCl = 2 × mole of Urea
mole of HCl = ...(i)
[We know , mole = M X V = N X n X V]
...as (i).
Therefore, Option(4) is correct.
View Full Answer(1)Crack CUET with india's "Best Teachers"
- HD Video Lectures
- Unlimited Mock Tests
- Faculty Support

The average molar mass of chlorine is 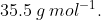 The ratio of
The ratio of 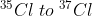 in naturally occurring chlorine is close to :
in naturally occurring chlorine is close to :
Option: 1 4:1
Option: 2 3:1
Option: 3 2:1
Option: 4 1:1
Given,
The average molar mass of chlorine is
Let , the ratio of in naturally occurring chlorine is close to x : y
Now, we know
So,
Therefore, the correct option is (2).
View Full Answer(1)
The minimum number of moles of O2 required for complete combustion of 1 mole of propane and 2 moles of butane is ______
Combustion reaction of 1 mole of propane and 2 moles of butane-

So, Total required mol of O2 = 5 + 13 = 18.
Ans = 18
View Full Answer(1)JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE


